Betadex Sulfobutyl Ether Sodium
Betadex Sulfobutyl Ether Sodium (SBECD) has been used in various routes of administration, including injection, oral, nasal, and ocular drug delivery. The modification with charged functional units enhances the binding affinity of cyclodextrin to guest molecules with opposite charges, thus exhibiting specific affinity towards nitrogen-containing drugs. This expanded binding capability allows for improved solubility, stability, and bioavailability of a wide range of pharmaceutical compounds. Furthermore, the inclusion complex formed between SBECD and nitrogen-containing drugs can protect the drugs from degradation and facilitate their controlled release, enhancing therapeutic outcomes.
-
Quick Details
-
Product Introduction
-
Quality advantage
-
Product Specification
-
Product Certificate
-
Application
-
Packing & Storage
-
Delivery
-
FAQ
| Name | Betadex Sulfobutyl Ether Sodium |
| CAS No. | 182410-00-0 |
| Molecular Formula | C42H70-nO35 (C4H8O3SNa)n |
| Molecular Weight | 1135+158n |
| Appearance | White powder, sweet, insipid and innocuous |
| Assay | 95.0%-105.0% |
| Classification | Injection Grade |
| Quality Standard | USP/EP |
| DMF No. | 030167 |
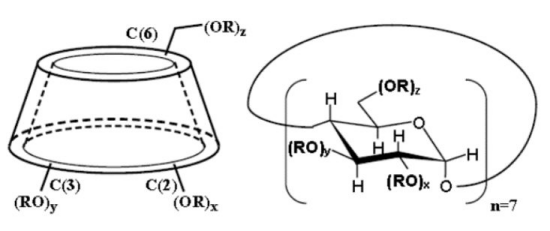
Molecular structuer:

Sulfobutyl ether β-cyclodextrin (SBECD) is a highly water-soluble anionic derivative of β-cyclodextrin. It is commonly used as pharmaceutical excipient or complexing agent in the pharmaceutical industry.
SBECD acts as a solubilizer and stabilizer for drugs with low water solubility. By forming inclusion complexes with drug molecules, the solubility, dissolution rate and bioavailability of the drug can be improved. The sulfobutyl ether group in SBECD is negatively charged and interacts with positively charged drug molecules, enhancing their hydrophobicity and solubility. This, in turn, can increase drug absorption, stability, and overall formulation properties.
Furthermore, the anionic nature of SBECD enables it to form non-covalent complexes with drug molecules, thereby improving stability, water solubility, and safety. It can help reduce kidney toxicity, prevent drug hemolysis, control drug release rates, and mask unpleasant odors associated with certain drugs.
The key index control is higher than the relevant quality standards
| ITEMS | RESULTS |
| 1,4-butane sultone | Not detected(<0.01ppm) |
| 4-hydroxybutane-1-sulfonic acid | Not detected(<0.0005%) |
| Bis(4-sulfobutyl) ether disodium | Not detected(<0.0005%) |
| Bacterial endotoxins | <0.005EU/mg |
| Clarity of solution and color | 30%(w/v) solution is clear and essentially free from particles of foreign matter. |
Hemolysis is better than the innovator drug.

Product advantages
1.Stable product quality and high production capacity.
2.Customizable according to requirements
3.Compliance with GMP standards
4.ISO certification attainment
Company advantages
1.Accepting OEM/ODM arrangements.
2.Providing free samples
3.13-year production and R&D experience.
4.Excellent after-sales service
5.Supporting various shipping options.
| Standard | USP |
| ITEMS | SPECIFICATION |
| Clarity of Solution | A 30%w/v solution in water is clear and essentially free from particles of foreign matter |
| Identification (IR) | Same absorption bands as USP betadex sulfobutyl ether sodium RS |
| Identification(Sodium) | Identify test are positive for Sodium |
| Identification(LC) | the retention time of the major peak of sample solution corresponds to the standard solution |
| Average Degree of Substitution | 6.2-6.9 |
| Water Solution pH | pH of 30%w/v solution in water is 4.0-6.8 |
| Water Content | ≤10.0% |
| β-Cyclodextrin Content | ≤0.1% |
| Sodium Chloride | ≤0.2% |
| 1,4-butane sultone | ≤0.5ppm |
| 4-Hydroxybutane-1-Sulfonic Acid | ≤0.09% |
| Disodium bis-(4-sulfobtyl) ether | ≤0.05% |
| Assay | 95.0%-105.0% |
| Bacterial Endotoxins | ≤10EU/g |
| Microbiology | |
| TAMC ( cfu/g) | ≤100 cfu/g |
| TYMC ( cfu/g) | ≤50 cfu/g |
| Escherichia Coli | Absence |

Betadex sulfobutyl ether sodium(SBEBCD) is a new type of anionic high soluble cyclodextrin derivatives, which can be used as a solubilizer, wetting agent, chelating agent (complexing agent) and polyvatent masking agent.
● Internal packing: packed PE bag, then put into aluminum foil bag
● Storage: Preserve well-closed containers protect from moisture.
● Label:according to customers’ request.
● Outer packing: packed in drum.
● Packing:100g/bag, 1kg/bag, 2kg/bag, 10kg/bag/drum, 20kg/bag/drum
1.Stock:Goods in stock
2.Delivery:Delivery goods within 1-3 workdays.
3.Loading location:factory of Binzhou Zhiyuan(China)
4.Shipping:FOB, CIF, CFR, DDU…..
Q:What are the advantages of Sulfobutyl beta Cyclodextrin Sodium Zhiyuan compared with other counterparts?
A:1、The hemolysis test and cytotoxicity test proved that there was no statistical difference in all data points between the Zhiyuan product and the original product.
2、Through UV absorbance comparative testing, the impurities in Zhiyuan products have reached the international original research level and are significantly better than those in the domestic industry.
3、Through comparative testing of the inclusion efficiency of posaconazole and voriconazole, it was found that Zhiyuan’s product has equivalent inclusion capabilities to the original research product and has good stability.
Q:What is the current progress of the joint application for Zhiyuan Sulfobutyl beta Cyclodextrin Sodium?
A:Zhiyuan’s Sulfobutyl beta Cyclodextrin Sodium and Simcere Pharmaceuticals made a joint application and state is Active. CDE No: F20190000285.
Q:What are the main sulfobutyl-β-cyclodextrin sodium inclusion complex injections currently on the FDA market?
A:voriconazole, Product name:Vfend
ziprasidone,Product name:Geodon
posaconazole,Product name:Noxafil