Hydroxypropyl-beta-cyclodextrin (HPBCD) Oral Grade
Hydroxypropyl-beta-cyclodextrin (HPBCD) has the characteristics of solubilization, antioxidant, odor removal, sustained release, and targeted delivery, and It has good solubility in water and can also be dissolved in 50% ethanol and methanol. There is a certain degree of relative hygroscopicity. However, the relative surface activity and hemolytic activity are relatively low. It is not irritating to muscles and is an ideal solubilizer and pharmaceutical excipient for injections.
-
Quick Details
-
Product Introduction
-
Quality advantage
-
Application
-
Packing & Storage
| Name | Hydroxypropyl-beta-cyclodextrin (HPBCD) |
| CAS No. | 128446-35-5 |
| Molecular Formula | C42H70-nO35(C3H7O)n |
| Molecular Weight | 1134.98+58n |
| Appearance | white powder, insipid, innocent |
| Assay | ≥98% |
| Classification | Oral Grade |
| quality standard | CP/USP/EP Standard |
| DMF No. | 030168 |
Examples:
Here are some examples of hydroxypropyl beta-cyclodextrin (HPβCD) applications:
Solubility Enhancement: HPβCD is widely used to improve the solubility of poorly soluble drugs. For example, the solubility of digoxin, a poorly soluble drug, can be increased from 0.07 mg/ml in water to 68 mg/ml in a 50% HPβCD solution.
Stability Improvement: HPβCD can enhance the stability of drugs. For instance, when estradiol is complexed with HPβCD, its degradation half-life at room temperature can be extended from 1.2 years to 4 years.
Reduction of Side Effects: HPβCD can help reduce the adverse effects of drugs. For example, the vasodilator drug nifedipine is poorly soluble in water and undergoes significant first-pass metabolism in the liver when taken orally. By formulating it as an injection using HPβCD, the drug’s absorption can be significantly increased, reducing damage to the gastrointestinal or muscular tissues.
Increased Bioavailability: HPβCD can enhance drug utilization. Steroid hormones, when complexed with HPβCD, not only exhibit high solubility and fast release rates but also result in approximately twice the drug levels in the bloodstream compared to the non-complexed form.
These examples illustrate the various benefits of using HPβCD in pharmaceutical applications. HPβCD offers advantages such as improved solubility, stability, reduced side effects, and increased drug bioavailability.
Product Introduction:
Hydroxypropyl-beta-cyclodextrin (HPBCD) are purified polydisperse products resulting from the controlled reaction of propylene oxide and native beta-cyclodextrin. Oral Grade Hydroxypropyl Beta Cyclodextrin is itself very soluble in water (greater than 500 mg/ml at room temperature compared to 18 mg/ml for beta-cyclodextrin), and is used for molecular encapsulation of a variety of sparingly water compounds to enhance the aqueous solubility of the encapsulated compound
HPBCD cyclodextrinis easily soluble in water, and its solubility is more than 50% (w/v) or even more than 75% (w/v) at room temperature. When its concentration is lower than 40%, it has good fluidity and is not viscous. When the solubility of insoluble drugs cannot be effectively increased by traditional methods, Hydroxypropyl-beta-cyclodextrin (HPBCD) can be considered to achieve the therapeutic effect. It solubilizes highly lipid soluble drugs, which can reduce stimulation and improve stability, but its solubilization effect is still limited. It has obvious solubilization effect only when the pH value range of the solution is 4.5-4.8.
Manufacturer information:
Hydroxypropyl-beta-cyclodextrin manufacturer-Shandong Binzhou Zhiyuan Biotechnology Co., Ltd which has completed registration with FDA, and the registration information is as follows:
★Company name: Shandong Binzhou Zhiyuan Biotechnology Co., Ltd
★FDA ESTABLISHMENT IDENTIFIER (FEI No.):3011680310
★ESTABLISHMENT ADDRESS: Boxing Economic Development Zone,, Binzhou, Shandong 256500, China (CHN)
★DUNS: 548171477
★We are a technological pharmaceutical excipient manufacturer focusing on the research, development, production and application of cyclodextrin derivatives and cyclodextrin inclusion compounds.
★We have a professional R&D team and sales team, 24-hour online customer service, and excellent quality control system and warehousing system


The hydroxypropyl beta-cyclodextrin produced by Zhiyuan Biotechnology has higher inclusion efficiency, smaller inclusion ratio and stronger inclusion capacity.

Oral Grade Hydroxypropyl Beta Cyclodextrin is easily soluble in water, and the solubility at room temperature is generally greater than 50g/100mL, and can even reach more than 80g/100mL. When the concentration is less than 40%, the fluidity is good and the viscosity is not large. Used in the pharmaceutical industry to improve the solubility of drugs without the use of organic solvents, surfactants and lipids. It can significantly increase the water solubility of poorly soluble drugs after being enveloped with it. After the drug forms a complex with hydroxypropyl-β-cyclodextrin(hbcd), its concentration increases linearly with the increase in hpbcd cyclodextrin concentration.
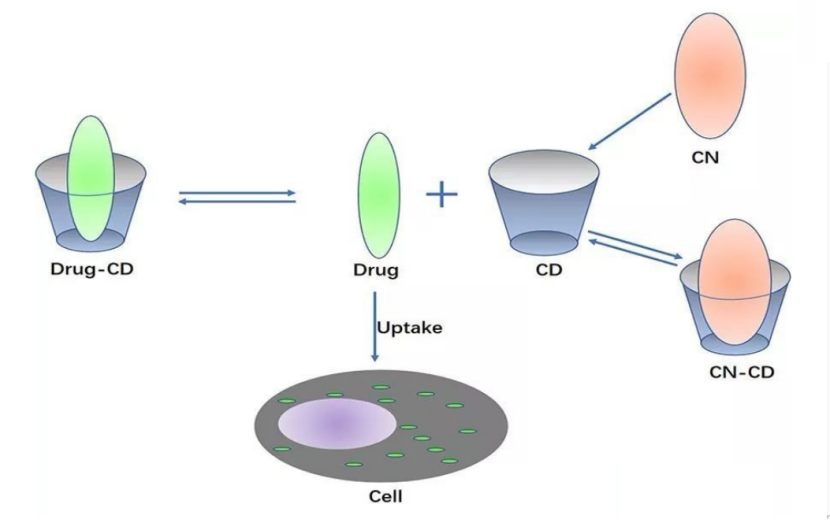
During the storage process of drugs, they will be affected by many factors such as light, heat, humidity, etc., which directly affect the quality of drugs. Therefore, stability is an important indicator. After the hydroxypropyl-beta-cyclodextrin (HPBCD) is incorporated into the drug, the drug enters the cavity, which can prevent the drug from volatilization, sublimation, oxidation and photolysis.
The drug enclosed with hpbcd cyclodextrin has fast dissolution rate and fast release, and can increase the absorption of the drug by the organism, which is beneficial to improve the bioavailability of the drug.
Complexing insoluble drugs and switching the route of administration can significantly reduce the toxicity and irritation of the drugs to the human body.
Hydroxypropyl-beta-cyclodextrin (HPBCD) can be used for oral drugs, mucosal drug delivery systems (including nasal mucosa, rectum, cornea, etc.), transdermal drug delivery systems, carriers of lipophilic targeted drugs, and can also be used as protein protective agent and stabilizer.
Due to the high hydrophilicity of hydroxypropyl betacyclodextrin, it is more conducive to the rapid dissolution of the drug in the gastrointestinal tract, improves its solubility in water, further improves the bioavailability of the drug, enhances the efficacy, and reduces the dosage.
Some drugs will degrade due to exposure to light, heat and oxygen, thereby losing some or even all of their efficacy, and cannot meet the normal requirement of maintaining stable properties within the drug’s validity period of 3 to 5 years. After inclusion using hydroxypropyl beta-cyclodextrin, the drug is sealed in the inner cavity of hydroxypropyl beta-cyclodextrin, which can effectively reduce photolysis, oxidation and thermal damage and help maintain drug stability. The pass rate increased nearly 4 times.
For drugs that are generally taken, the inclusion effect of hydroxypropyl betacyclodextrin on drugs can achieve four types of controlled release effects, namely immediate release, delayed release (time-controlled release), extended release and controlled release.
Hydroxypropyl cyclodextrin can enhance the corneal permeability of drugs. For example, hydroxypropyl beta cyclodextrin can increase the corneal penetration rate of pilocarpine nitrate by nearly 4 times.
● Packing: 1kg/2kg/10kg/20kg/drum or carton
● Storage: Preserve well-closed containers protect from moisture.
● Inner packaging: sterile PE bag, aluminum foil bag;
● Outer packaging: full carton;